Radio, T.V., microwave, visible light, u.v. light, x-rays,
![]() -rays are
all electromagnetic radiation and differ only in wavelength.
Electromagnetic radiation may have both beneficial and harmful effects:
e.g. u.v. light absorbed by the skin can supply needed vitamin D, but
excess u.v. radiation accounts for much skin cancer.
X-rays and
-rays are
all electromagnetic radiation and differ only in wavelength.
Electromagnetic radiation may have both beneficial and harmful effects:
e.g. u.v. light absorbed by the skin can supply needed vitamin D, but
excess u.v. radiation accounts for much skin cancer.
X-rays and ![]() -rays are more penetrating and so can
affect tissue below the skin.
-rays are more penetrating and so can
affect tissue below the skin.
Besides electromagnetic radiation one has high velocity charged
(and neutral) particles.
From naturally radioactive materials, the charged particles
are either high
speed electrons (![]() 's, beta rays) or alpha particles (
's, beta rays) or alpha particles (![]() 's,
the nuclei of helium atoms).
Both types are rather easily stopped by a small thickness of
matter e.g.
's,
the nuclei of helium atoms).
Both types are rather easily stopped by a small thickness of
matter e.g. ![]() 1.8 mm Al stops 1.17 MeV
1.8 mm Al stops 1.17 MeV ![]() 's from RaE,
and
's from RaE,
and ![]() 0.06 mm Al
stops 5.3 Mev
0.06 mm Al
stops 5.3 Mev ![]() 's from Po. Hence natural radioactivities
are normally of little concern unless
the parent nucleus has been inhaled or ingested in the body. The biological
effects of neutral particles (e.g. neutrons and neutrinos)
from naturally radioactive materials are normally negligible.
's from Po. Hence natural radioactivities
are normally of little concern unless
the parent nucleus has been inhaled or ingested in the body. The biological
effects of neutral particles (e.g. neutrons and neutrinos)
from naturally radioactive materials are normally negligible.
-
- Radioactivity unit:
- 1 becquerel (Bq) = 1 disintegration/s
 27 pCi (picoCurie)
27 pCi (picoCurie)
- Radiation dose
- is the radiant energy absorbed per unit mass.
- Dose UNITS:
- 0.01 J of radiation absorbed/kg of
mass = 1 rad
1 J of radiation absorbed/kg of mass = 1 gray (abbreviated Gy) - Dose Equivalent:
- includes the long term relative
biological
effects of different types of radiation. The original unit was the
rem (rad equivalent man),
but the now recommended S.I. unit is the
sievert (abbreviated Sv) with:
1 Sv = 100 rem = 10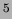 mrem
mrem
and so: 1 mSv = 100 mrem